| contact us |
| why choose Chiralabs? |
Circular Dichroism Applications:
Protein Structure Analysis: Near-UV
Spectral Characteristics
In the near-UV wavelength region (240-360nm) the major chromophores generating CD features are the chromophoric-aromatic side chains of tryptophan, tyrosine and phenylalanine together with the disulphide linkages, if present, as depicted below.
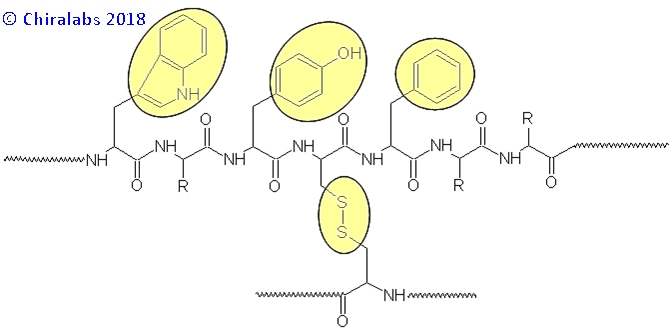
Each of these contributes at different positions in the near-UV region, allowing their contributions to be discerned from each other to some degree. The figure below gives an example of the near-UV CD spectra of a IgG monoclonal antibody repeated over many days, where the contributions and reproducibility can be seen.
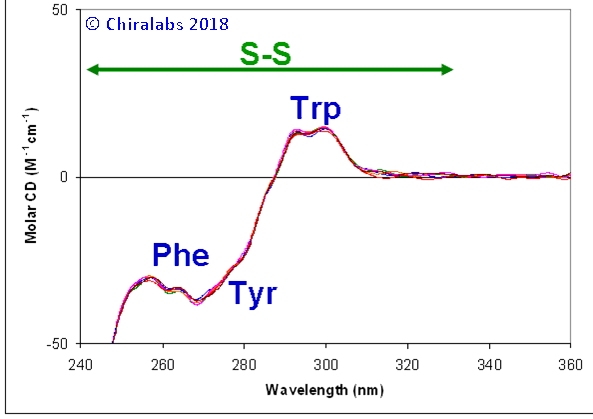
The disulphide contributions are typically rather broad and underlie the narrower features of the aromatics; however, they often extend to 330nm, beyond where tryptophan and the other aromatics contribute. Their contribution to the spectrum is dependent in large part on the torsion angles of the disulphide bonds; changes in a spectrum around 310-330nm are often related to a change in the disulphide bonding pattern (if they extend to 360nm or beyond it is likely some other cause is responsible, such as scattering, other intrinsic chromophores or anomalous chromophores).
The aromatic bands are most easily distinguished at 285-310nm for tryptophan, 275-285nm for tyrosine and 255-270nm for phenylalanine. In each case their contribution represents the chiral influence of their environments, changing magnitude, sign and, to lesser extent, precise position with changing environment. A change in any of the respective bands can then be related to a location in the protein where these amino acid residues occur.
Solution & Spectroscopic Conditions
Given that the aromatic residues and disulphides are usually in the minority compared to the number of peptide linkages in a natural protein, the net absorbance of a protein solution in the near-UV region is invariably weaker than that in the far-UV region. Consequently, higher concentrations or longer pathlengths are employed to meet optimal spectroscopic conditions in the near-UV region. Again, an absorbance of ca. 0.87AU is optimal and, for most proteins, can be met by a 1mg/ml solution in a 1cm pathlength cell, although proteins rich in tryptophan may require a reduction in concentration or pathlength accordingly. If the amino acid composition of the protein is known, the absorbance at ca. 280nm can be estimated from the typical molar absorbances of tryptophan, tyrosine and disulphide linkages in native proteins at this wavelength.
Spectral Processing
Essentially all the same processes and concerns described for the far-UV protein page apply to the near-UV region also. However, one important difference is in the definition of molar absorbance and molar CD. Because the relevant chromophores are no longer the peptide linkages, a more conventional definition based on normalisation by the pathlength (ℓ in cm) and “whole molecule” concentration of the protein, is adopted for the calculation of the molar CD and molar absorbance.
Interpretation of NUV CD Spectra
Unlike the far-UV region CD, the spectra cannot be readily interpreted in terms of any specific secondary or tertiary structure. Instead, the spectra are employed as a specific, sensitive fingerprint for the environments of the near-UV chromophores, such that a change in their local environment, whether it be due to a secondary or tertiary structural change or the binding of a moiety, can often be detected.
For example, the figure below depicts a series of near-UV CD spectra over a thermal denaturing profile of a IgG monoclonal antibody preparation, from which various features can be deduced. At temperatures above 35°C a shift in the “apparent” CD in the 340-360nm region occurs; this is due to a degree of scattering that begins to occur (as evidence by the accompanying UV absorbance spectra that were acquired), likely related to the precipitation of one minor form or impurity out of the population of species in the sample. Distinguishable spectral changes in the aromatic contributions start happening at ca. 40°C, particularly in the region where phenylalanines give features. At 65-70°C, there a dramatic loss in magnitude around 250-280nm, but the fine features of the phenylalanines remain, suggesting it is the underlying disulphides that have begun to move; concomitantly, the structure of the tryptophan bands changes. All these precede the catastrophic denaturation and precipitation at 80°C.
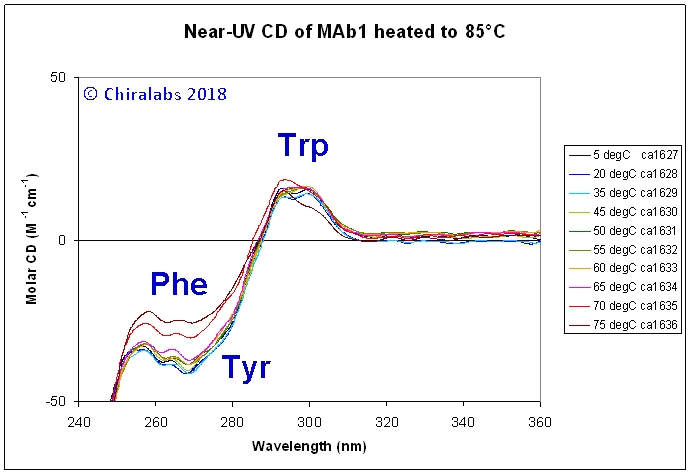
It may be noted that most proteins do not thermally denature to an “irregular” structure, but rather to a new folded structure that, in many cases, is sticky, aggregates readily and precipitates. Moreover, they exhibit a series of structural transitions at lower temperatures preceding their final “denaturing”. This can be successfully followed both by far-UV and near-UV CD to gain insight into the process from a secondary structural (far-UV) and local environment (near-UV) perspective. It is often the case that the two spectral regions show differing events with temperature. For example, a loosening of tertiary structure is often reflected in a change in the near-UV spectra, but the far-UV spectra remain unperturbed, indicating the main secondary structure is preserved.
- please contact us to see how we may enhance your analytical capabilities or help solve your analysis problems.
References
For further literature see our Literature page